The focus of this learning module for health educators is the management of diabetes in stages 4 and primarily stage 5 chronic kidney disease (CKD). Diabetes management in stages 1-3 CKD differs little from that of diabetes management without renal disease.
- Stage 1: GFR > 90
- Stage 2: GFR 60-89
- Stage 3: GFR 30-59
- Stage 4: GFR 15-29
- Stage 5: GFR < 15 or dialysis (End Stage Renal Disease – ESRD)
Diabetes is the most common cause of end-stage renal disease (ESRD) and dialysis, but individuals can have CKD and ESRD from other causes and have diabetes as an additional comorbidity.
Topics
- Factors Affecting Glucose in Stages 4 - 5 CKD
- Factors Predisposing to Hypoglycemia
- Evaluation of Glycemic Control
- Glycemic Targets
- Nutrition
- Non-insulin Antihyperglycemic Agents
- Starting Insulin Doses in ESRD
- Kidney Replacement Therapies
- Hyperosmotic Hyperglycemic State (HHS) and Diabetic Ketoacidosis (DKA)
Factors Affecting Glucose in Stages 4 - 5 CKD
Diabetes is the most common cause of end-stage renal disease (ESRD) and dialysis, yet there is limited data addressing how to best treat diabetes in this population. ESRD significantly affects glycemic control, standard methods for estimating glycemic control, and the excretion of the medications used to treat diabetes (1). Moreover, ESRD and dialysis exert opposing forces on insulin secretion, action and metabolism (3).
Role of the kidney in glucose homeostasis
The two key organs that play a role in glucose production (gluconeogenesis) are the kidney and the liver. The kidney is responsible for up to 40% of the glucose produced through gluconeogenesis in the fasting state (4). Insulin suppresses renal glucose production and increases glucose uptake, but the kidney has no significant glycogen stores. Different from hepatic gluconeogenesis, glucagon has minimal effect on renal gluconeogenesis.
The kidney plays an additional role in glucose uptake, filtration, and reabsorption (4). Glucose is freely filtered at the glomerulus and reabsorbed completely in the proximal tubule via an insulin independent process (SGLT2/SGLT1 glucose transporters). Once the maximal reabsorptive capacity of the proximal tubule is reached (Tm), glycosuria (glucose in the urine) occurs. The maximal reabsorptive capacity is dependent on the estimated glomerular filtration rate (eGFR) and glucose loads; as the eGFR declines there is a reduction in glucose reabsorption.
Insulin dynamics in CKD
Insulin is metabolized primarily by the liver and the kidney. About 40-50% of insulin clearance occurs in the kidney. However, exogenous insulin (as used in patients with diabetes) is NOT metabolized by the liver, so escapes this first-pass metabolism. This increases the role the kidney plays in insulin clearance (5).
Additionally, as eGFR decreases to 15-20 mL/min, the clearance of insulin decreases accordingly. This results in an increase in the duration of action of insulin and may contribute to hypoglycemic episodes. As patients progress to ESRD, their insulin requirements drop. Some patients with Type 2 diabetes may no longer require insulin, while others may not need evening basal insulin (6).
Metabolic abnormalities
CKD presents with multiple metabolic abnormalities including uremia and metabolic acidosis, hyperparathyroidism, and protein energy malnutrition; all of which can affect insulin dynamics.
- Uremia and metabolic acidosis and pro-inflammatory mediators: these contribute to insulin resistance (IR) which decreases insulin-mediated uptake of glucose by muscle and adipose tissue, contributing to hyperglycemia (4).
- Parathyroid hormone (PTH): PTH decreases insulin secretion contributing to hyperglycemia. In patients with progressive decline in their renal function, it is common to see a rise in serum parathyroid hormone concentrations (secondary hyperparathyroidism) due to an inability of the kidney to produce active vitamin D (1, 25 dihydroxyvitamin D) (4).
- Protein energy malnutrition: This is common in dialysis patients as there is a state of relative deficit of nutritional intake as compared to nutritional demand. This can increase IR through increases in inflammatory cytokines (4).
Factors predisposing to hypoglycemia
There are several factors in ESRD which increase the risk of hypoglycemia. These include reduced insulin clearance, reduced renal gluconeogenesis (glucose production), altered pharmacokinetics of glucose lowering medications, and decreased hepatic metabolism of endogenous insulin because of uremia (3, 7). Hypoglycemia in this group of patients can also be secondary to non-diabetes related causes including co-existing adrenal insufficiency, malnutrition, organ failure, infection and other medications.
Complications of diabetes such as gastroparesis may also increase the risk of hypoglycemia as well as contribute to significant variability in blood glucose levels (7).
Taken together, individuals with diabetes and ESRD are at significantly increased risk of hypoglycemia, specifically if their treatment regime contains an insulin secretagogue or insulin. They are also at risk for hypoglycemia unawareness.
CKD Factors affecting blood glucose (4)
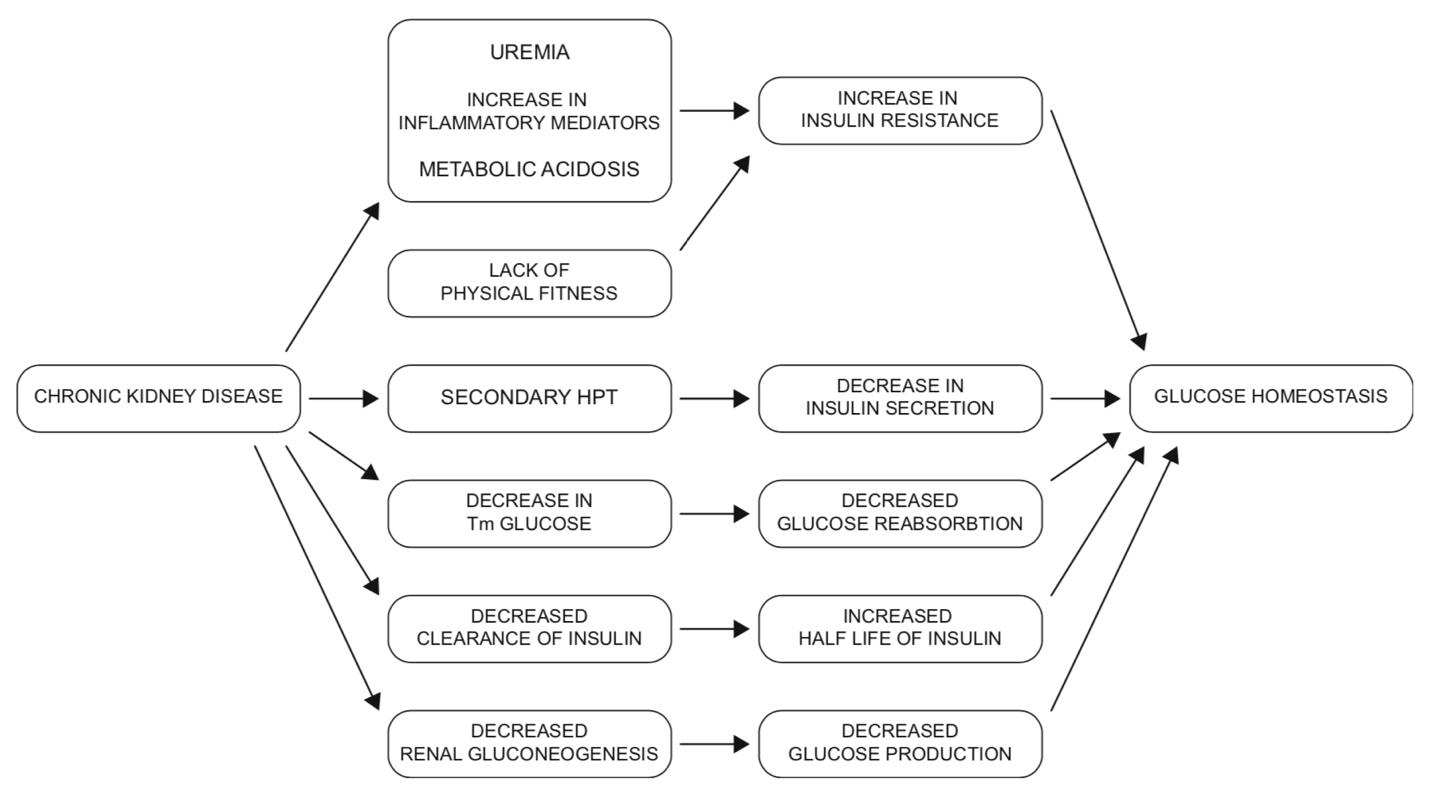
Factors affecting glucose homeostasis in dialysis patients (4)
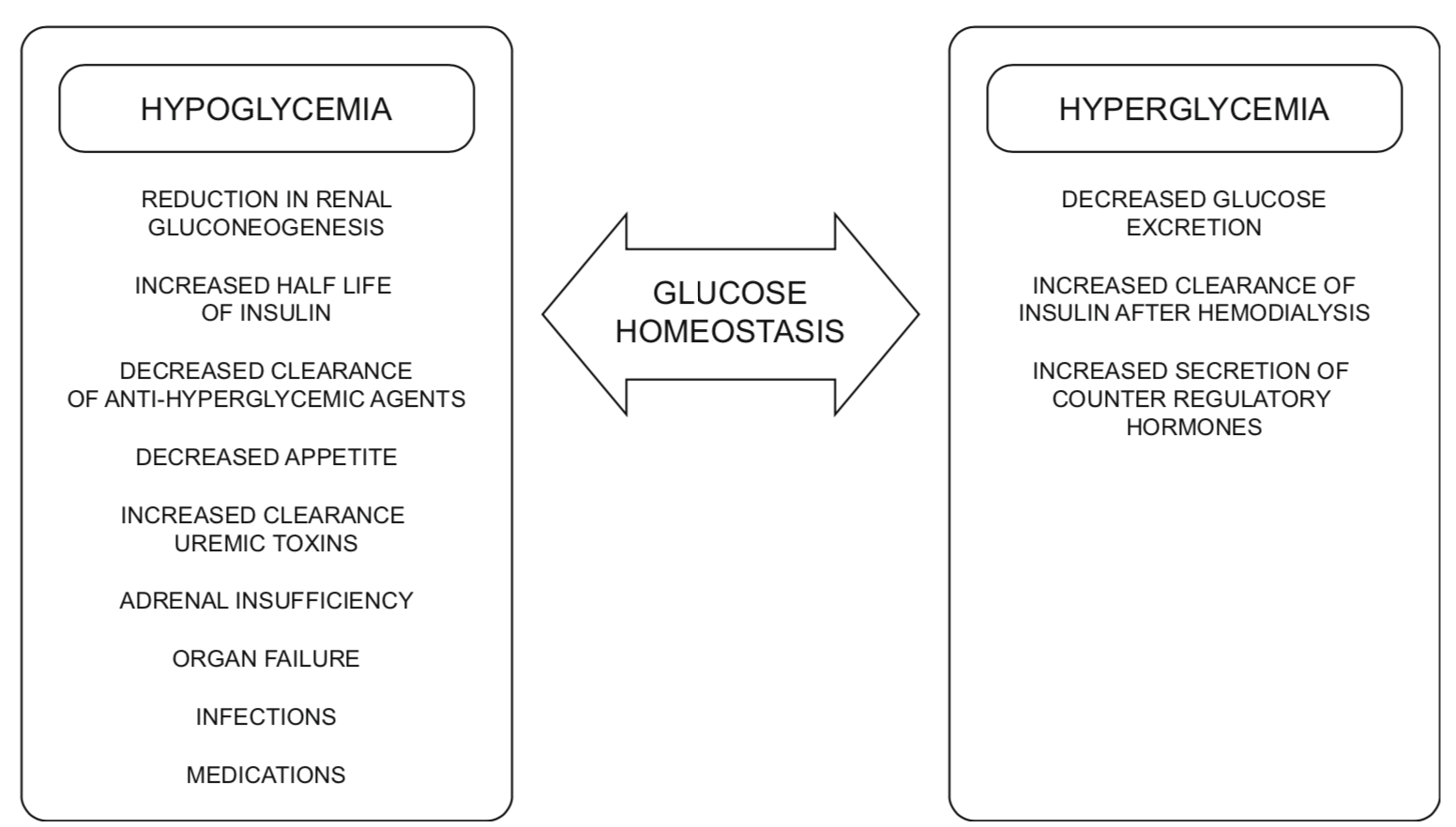
Evaluation of Glycemic Control
A1c
Healthcare providers must be aware of the limitations of A1c as a marker of glycemic control in individuals with ESRD. Overestimation of the HgA1c can occur with factors that reduce RBC “turnover,” such as iron deficiency, vitamin B12 deficiency or decreased erythropoiesis. Conversely, when individuals with ESRD are administered iron or erythropoietin, this may result in an increase in RBC number, less glycosylation and a subsequent underestimation of the A1c. Chronic kidney disease itself and hemodialysis may reduce RBC lifespan, underestimating the A1c.
Finally, blood urea nitrogen, which is increased in ESRD, causes formation of carbamylated hemoglobin. Carbamylated hemoglobin is indistinguishable from glycated hemoglobin resulting in overestimation of HgA1c in patients with ESRD. It is important to know your local hemoglobin A1c assay. Of note, Alberta Precision Laboratory A1c assay is not affected by carbamylated Hb.
Factors that affect A1C: Diabetes Canada 2018 Guideline: Monitoring Glycemic Control
Other measures of glycemic control
Glycated fructosamine and glycated albumin are other measures of glycemic control. However, they may be affected by conditions that alter protein metabolism, which is seen with ESRD especially in patients with nephrotic syndrome, those receiving peritoneal dialysis, and with malnutrition. There are no internationally accepted methods or reference ranges that have been sufficiently validated. Consequently, they are not recommended as measures of glycemic control in ESRD.
Home glucose monitoring
- Capillary Blood Glucose (CBG): Home glucose monitoring plays a very important role in assessing glycemic control and guiding therapy given the limitations of HgA1c. Glucose targets must be individualized. In general, CBG < 8.0 mmol/L before meals and < 12.0 mmol/L 2 hour after meals, are often considered, avoiding hypoglycemia and significant hyperglycemia.
- Continuous glucose monitors (CGM): Although CGM are not currently indicated for use in patients on dialysis, they are not contraindicated and are increasingly seen as valuable. CGM can provide glucose trend data, during and after dialysis, to inform dose adjustment of diabetes medications. Icodextrin (Extraneal), used in peritoneal dialysis (PD), is not a known interfering agent for CGMs. For all patients on dialysis ,continue to suggest capillary blood glucose checks if sensor results don't match patient symptoms. (Important note: The time lag between capillary blood glucose and interstitial glucose may be increased in ESRD, which reduces the ability of CGM to detect hypoglycemia.)
Please note that home monitors may be affected by a particular peritoneal dialysate solution, icodextrin (Extraneal®). The following links provide a list of glucose monitors approved in Canada that are compatible with this dialysate:
- https://www.glucosesafety.com/ca/en/index.html
- https://www.glucosesafety.com/ca/en/downloads/2021-12-07-CSGML_Canada_English_2021.pdf
Glycemic Targets
Despite the limitations outlined in the previous section, it is still recommended to use A1c and rely on trends in the results.
There is limited data available regarding optimal A1c targets in ESRD. The relationship between A1C and mortality in non-dialysis and dialysis patients has been shown to be U-shaped, with mortality lowest in the 7.0-7.9% range; indicating it is potentially beneficial to keep the A1C within an optimal target (4). The suggested goal is an A1c in the 7.1 – 8.5% range, with targets being individualized (1). The primary goal is to avoid hypoglycemia and significant hyperglycemia. Most patients with ESRD have co-morbidities that would suggest this less aggressive A1c target.
Please see 2018 Diabetes Canada Quick Reference Guide for Targets for glycemic control.
Nutrition
- Referral to a Registered Dietitian for nutrition counselling to ensure nutritional adequacy of the diet is recommended for patients with declining kidney function (see Renal Nutrition Pathway DCC).
- Please see the specific factors related to HD and PD (links following). Here are some additional nutritional considerations:
- A bedtime snack is often recommended in stages 4-5 CKD for individuals on insulin or insulin secretagogues, even in the absence of injected evening basal insulin, to prevent hypoglycemia.
- Glucose from dialysate can impact blood glucose levels and weight, as previously discussed. This is of greater concern in peritoneal dialysis.
- Patients with gastroparesis should be encouraged to eat smaller, frequent, low-fat, and low-fibre meals.
- The management of ESRD will require that some foods or supplements be limited in sodium, potassium, phosphorus, fluid, or protein content depending on individual patient laboratory results.
Non-insulin Antihypergycemic Agents
Medications to manage glycemia should be prescribed and adjusted to suit individual patient renal function, glucose patterns, lifestyle, and comorbidities. As renal function declines, some diabetes medications require a dose reduction and others may need to be discontinued. Thus, antihyperglycemic agents need to be reassessed as patients progress through all stages of CKD and with eventual initiation of kidney replacement therapies.
-
In stage 5D CKD, sulfonylureas are usually discontinued given their reduced clearance and risk of hypoglycemia.
-
Select antihyperglycemic agents may be continued depending on the eGFR and other comorbidities; these include SGLT2 inhibitors, GLP-1 receptor agonists, DPP4 inhibitors, repaglinide and metformin, if deemed appropriate in individual patients.
-
SGLT2 inhibitors and GLP-1 receptor agonists may be continued for their cardiovascular and/or renoprotective benefits.
-
SGLT2 inhibitors have reduced glucose lowering capacity below a GFR of 45mL/min.
Diabetes Canada resources with information on antihyperglycemic medication considerations in renal impairment:
Starting Insulin Doses (ESRD):
Insulin doses are lower in patients with ESRD than in those with higher eGFR. Here are some suggested guidelines for insulin dose calculations in these patients:
-
For full BBIT: Total Daily Dose (TDD) of 0.15 units/kg/day
-
Basal insulin start: 0.1 unit/kg
-
Bolus insulin addition: 0.05 units/kg/meal
-
Please see bbit.ca, “How to BBIT (For Prescribers)” document, Special Populations, Suggestions for patients with renal impairment and the Diabetes Educator Calgary website, Insulin Formulas page.
Kidney Replacement Therapies – types and considerations
In individuals with ESRD, kidney replacement therapies, hemodialysis and peritoneal dialysis, are lifesaving. ESRD and dialysis exert opposing forces on insulin secretion, action, and metabolism, with the potential to create unpredictable serum glucose values. Dialysis may also alter the pharmacokinetics of diabetes medications.
Hemodialysis (HD)
Hemodialysis is administered in a dialysis unit or at home, often 3 days per week and for 4 hour runs. Hemodialysis prescriptions are individualized, and patients may be receiving hemodialysis more or less frequently, with some patients undergoing nocturnal treatments for 6-8 hours several days per week.
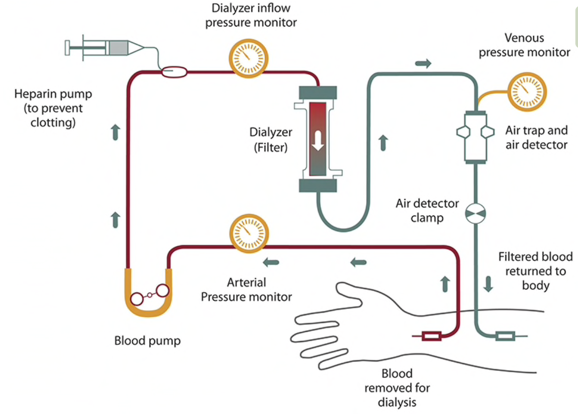
To learn more or to view this figure in a larger format, please go to National Institute of Diabetes and Digestive and Kidney Diseases, NIH.
Glycemic Effects (HD):
HD can predispose to both hyperglycemia and hypoglycemia due to its multiple effects on blood glucose, medication clearance and insulin dynamics. Factors affecting glucose levels include the following (4):
-
HD can remove insulin from the patient
-
HD baths are made of varying concentrations of dextrose. Using dextrose-free dialysate can result in hypoglycemia in up to 40% of patients. Most HD baths contain a dextrose concentration of 5.5 - 11.1 mmol/L, which avoids hypoglycemia during an HD run. However, the dialysate is selected most often based on the potassium and other electrolyte content of the bath, not on the glucose concentration.
-
Uremia decreases with HD, which increases insulin sensitivity late in the HD run and after the run.
The net effect is an increased risk of hypoglycemia on dialysis days. For patients treated with insulin, doses will vary between dialysis and non-dialysis days.
Insulin Administration and HD
Renal clinicians monitor blood glucose levels carefully before, during and after hemodialysis runs and usually suggest snacks as required.
A basal bolus insulin therapy (BBIT) regimen with regular home glucose monitoring may be considered the safest regimen, with analogue insulin chosen over intermediate insulin (NPH) when recurrent hypoglycemia occurs (1). For those unable or unwilling to self-inject insulin, there have been case reports using three times per week of long-acting insulin at the end of dialysis. Further research is needed on the ultra-long acting insulins (i.e. degludec) (9). Biphasic (twice daily pre-mix) insulins may be difficult to manage in those receiving hemodialysis because of the changes in diet, glucose levels, and activity restriction between dialysis and non-dialysis days. However, patients stable on these regimens may not require or want a change.
Insulin pump therapy (including automated insulin delivery systems) may be considered for people with type 1 or type 3c diabetes, to meet individual insulin requirements.
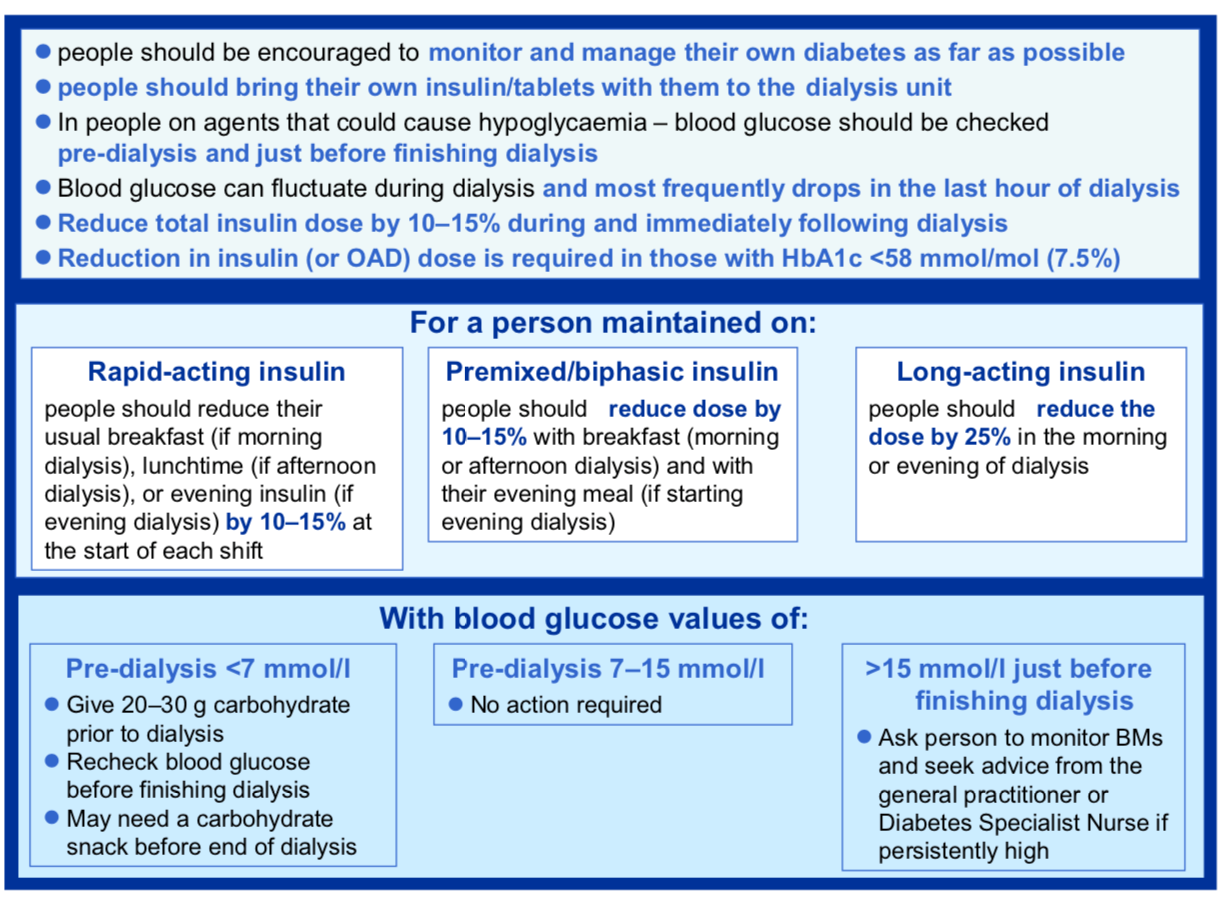
Typically, a 10-15% reduction in total daily dose of insulin on hemodialysis days may be required to avoid hypoglycemia. Below is an initial template for insulin dose adjustments around dialysis. This should be further adjusted based on individual patient factors (1).
Insulin doses will vary between dialysis and non-dialysis days, with lower insulin requirements on hemodialysis days. The same basic principles of insulin adjustment apply with pattern recognitions informing any changes in insulin brand, timing and dosing.
Insulin management in patients with DM on dialysis (1)
Nutrition and Appetite in HD:
-
Snacks are often necessary during and after an HD run. Some patients may be encouraged to eat during hemodialysis to prevent low blood sugars. Patients are advised to carry food and treatment for hypoglycemia with them on Calgary Transit Access or vehicle after a dialysis run.
-
Patients with gastroparesis should have a low-fat and low-fibre meal prior to hemodialysis, as well as bring their own snacks to hemodialysis.
-
Patients often have poorer appetites and intake before the HD run due to uremia, and significantly improved appetites after the run. As a result, many patients eat large meals after the HD run as they are feeling better. Rapid or short acting glucose-lowering medications may need to be adjusted/increased for that larger meal post-dialysis (1).
Peritoneal Dialysis (PD)
There are two types of peritoneal dialysis, continuous cycler peritoneal dialysis (CCPD) and continuous ambulatory peritoneal dialysis (CAPD).
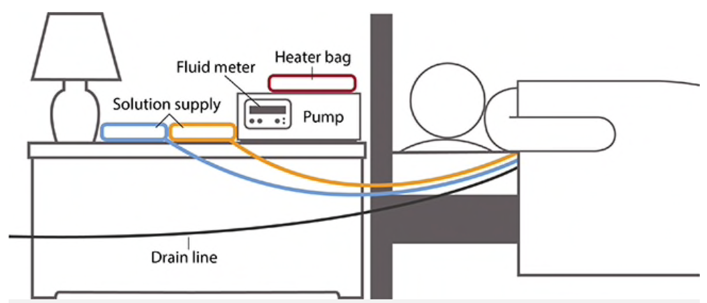
CCPD: either a dialysis solution is used during the day (the patient is ‘wet’), or no solution is used (‘dry’), and at night, the PD catheter is connected to a dialysis machine called a cycler, which loads various PD dialysis fluids during the night.
CAPD: involves 3 – 4 daytime exchanges and one overnight exchange
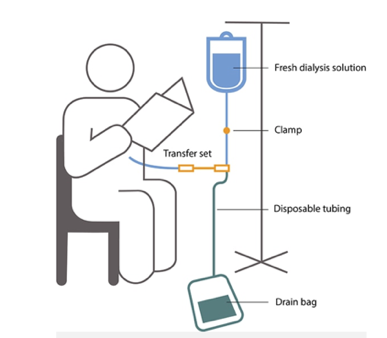
To learn more or to view these figures, please go to National Institute of Diabetes and Digestive and Kidney Diseases, NIH.
Dialysates used for PD typically contain dextrose as the osmotic agent used to draw out fluid from the body, which can contribute to hyperglycemia.
The following dextrose concentrations are available: 0.5%,1.5%, 2.5%, 4.25%.
-
1.5% (83.3 mmol/L) is most common choice
-
2.5% (139 mmol/L) is ordered if the patient is above their target weight, removing up to 500 ml of fluid per dwell
-
4.25% (194.6 mmol/L) will remove larger amounts of fluid from the patient (up to 1000 ml) per dwell
Alternatively, there are PD solutions that use carbohydrate polymers icodextrin (Extraneal®) as the osmotic agent which does not cause hyperglycemia. Extraneal may be used for one of the 3-4 bags during the day or overnight.
The dextrose absorption from the dialysate can be variable due to individual peritoneal membrane permeability characteristics that affect treatment prescriptions, dextrose concentrations, dwell time and the number and volume of exchanges. Blood glucose levels can alter with peritonitis (infection or inflammation) and with obstructed drainage of dialysate. Constipation can impair drainage of dialysate.
PD in the Calgary Zone:
-
Most PD patients use the cycler machine at bedtime which does a series of exchanges with mostly a 1.5% solution and the 7.5% Extraneal solution as a last fill which stays in the abdomen throughout the day (for 10-12 hours). This predisposes to hyperglycemia overnight.
-
Some PD patients do manual exchanges instead with usually 3 exchanges/day (3-4 hour dwell time in the morning, noon and afternoon) and then 7.5% Extraneal at night while sleeping, which predisposes to hyperglycemia during the day.
Insulin Administration and PD
There is very limited data suggesting the best insulin regimen for patients on PD. In some centers, insulin is added to the PD glucose solution but can be associated with bacterial complications and other safety concerns; this is not the typical practice in the Calgary Zone.
A basal bolus insulin therapy (BBIT) regimen has been studied and found safe and effective (16).
Other possible regimens include (4):
-
Patients with type 1 diabetes or those with type 2 on multiple doses of insulin (MDI): some version of the typical subcutaneous twice daily basal and three times daily bolus injection regimen, but the two basal doses may not be equal (e.g. patients who use cycling machines to perform automatic overnight exchanges, will often have a higher basal dose at night and a much lower basal dose in the morning).
-
Insulin pump therapy (including automated insulin delivery systems) may be considered for people with type 1 or type 3c diabetes, to meet individual insulin requirements.
-
Patients with type 2 diabetes who use cycling machines to perform automatic overnight exchanges, may only need a single subcutaneous basal dose of insulin in the evening, and it is possible they may need only low-dose or no prandial insulin or repaglinide during the day.
-
Some patients with type 2 diabetes may only require a subcutaneous twice daily NPH regimen, with doses varying based on type of dialysis (CCPD vs. CAPD).
-
In type 2 diabetes, repaglinide may be used for meals or for CAPD/TID dialysate exchanges.
With CCPD, the patient may require basal insulin 2 – 3 hours before starting the cycler (usually at night). This allows for the onset of insulin action close to the time that the cycler starts. Long-acting insulin may continue to work after the cycler (and the glucose infusion) stops. Hypoglycemia may be avoided by directing the individual to have small amount of carbohydrate (e.g. juice) before rising and becoming more active.
Nutrition and appetite in PD
-
Meals and carbohydrate intake may vary due to decreased hunger secondary to abdominal fluid volume, increased caloric intake from the dialysate and, early on, to lingering symptoms of uremia.
-
The glucose absorption can be variable due to individual peritoneal membrane permeability characteristics that affect treatment prescriptions, dextrose concentrations, dwell time and number and volume of exchanges.
-
Calories from glucose absorption should be considered when calculating energy requirements.
To inform diabetes medication adjustments, especially insulin doses for patients on PD, it is essential to know which dialysates are being used and when, know the patient’s nutritional intake/timing and to encourage frequent monitoring of glucose. Also consider other factors that can alter the glucose levels including peritonitis (infection or inflammation) and obstructed drainage of dialysate (constipation can impair drainage of dialysate).
The same basic principles of insulin adjustment apply with pattern recognitions informing any changes in insulin brand, timing and dosing.
Transitioning from PD to HD
Patients must be monitored closely. There is an increased risk of hypoglycemia secondary to decreased exposure to dextrose-containing peritoneal dialysis fluids. Insulin and antihyperglycemic therapy should be modified accordingly.
Hyperosmotic Hyperglycemic State (HHS) and Diabetic Ketoacidosis (DKA)
Many individuals with ESRD are either anuric or oliguric (produce no to little urine), so severe dehydration and hyponatremia tends not to occur. But a major concern in these patients is that, due to extracellular hypertonicity, potassium and fluid may shift extracellularly to cause hyperkalemia and acute intravascular volume expansion and for patients with Type 1 diabetes, worsening metabolic acidosis. Insulin is the mainstay of treatment of DKA (14, 15).
Patients with stage 5D CKD (dialysis) are often on fluid restrictions. Therefore, advising increased fluid intake for the prevention of DKA should be approached with caution in patients with Type 1 diabetes. Insulin therapy adherence in this population is encouraged.
Diabetes educators are encouraged to contact the endocrinologist or nephrologist involved for appropriate instructions for DKA prevention in patients with Type 1 diabetes on dialysis.
References
-
Frankel AH, Kazempour-Ardebili S, Bedi R, Chowdhury TA, De P, El-Sherbini N, et al. Management of adults with diabetes on the haemodialysis unit: summary of guidance from the Joint British Diabetes Societies and the Renal Association. Diabet Med. 2018;35(8):1018-26.
-
Gregory S, Jenkins K. Managing care for people with diabetes undergoing dialysis. J Ren Care. 2019;45(1):59-67.
-
Shrishrimal K, Hart P, Michota F. Managing diabetes in hemodialysis patients: observations and recommendations. Cleve Clin J Med. 2009;76(11):649-55.
-
Garla V, Yanes-Cardozo L, Lien LF. Current therapeutic approaches in the management of hyperglycemia in chronic renal disease. Rev Endocr Metab Disord. 2017;18(1):5-19.
-
Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev. 1998;19(5):608-24.
-
Alsahli M, Gerich JE. Hypoglycemia, chronic kidney disease, and diabetes mellitus. Mayo Clin Proc. 2014;89(11):1564-71.
-
Abe M, Kalantar-Zadeh K. Haemodialysis-induced hypoglycaemia and glycaemic disarrays. Nature reviews Nephrology. 2015;11(5):302-13.
-
Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011;154(8):554-9.
-
Oishi A, Makita N, Manaka K, Mitani K, Tomita H, Iiri T. Successful glycemic control with three times a week degludec injection by medical staff for an elderly hemodialysis patient with type 2 diabet Diabetology International (2016) 7:95–99.
-
Ng JM, Cooke M, Bhandari S, Atkin SL, Kilpatrick ES. The effect of iron and erythropoietin treatment on the A1C of patients with diabetes and chronic kidney disease. Diabetes Care. 2010;33(11):2310-3.
-
Bomholt T, Adrian T, Nørgaard K, Ranjan AG, Almdal T, Larsson A, et al. The Use of HbA1c, Glycated Albumin and Continuous Glucose Monitoring to Assess Glucose Control in the Chronic Kidney Disease Population Including Dialysis. Nephron. 2021;145(1):14-19.
-
Wang F, Wang D, Lyu XL, Sun XM, Duan BH. Continuous glucose monitoring in diabetes patients with chronic kidney disease on dialysis: a meta-analysis Minerva Endocrinol. 2020 Dec 3.
-
Alalawi F, Bashier A. Management of diabetes mellitus in dialysis patients: Obstacles and challenges. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 15 (2021) 1025-1036.
-
Ben-David E, Hull R, Banerjee D. Diabetes mellitus in dialysis and renal transplantation. Therapeutic Advances in Endocrinology and Metabolism. 2021 Oct 5 (12); 1-16.
-
Seddike, et.al. Challenges in management of diabetic ketoacidosis in hemodialysis patients, case presentation and review of literature. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2019 13;2481-87.
-
Gomez AM, et.al. Impact of a Basal-Bolus Insulin Regimen on Metabolic Control and Risk of Hypoglycemia in Patients With Diabetes Undergoing Peritoneal Dialysis. Journal of Diabetes Science and Technology 2018, Vol. 12(1) 129–
Additional Resources and Reading
Diabetes Canada 2018 Guidelines chapter: Chronic Kidney Disease in Diabetes
Management of adults with diabetes on the haemodialysis unit Joint British Diabetes Societies (JBDS) for Inpatient Care Group
Nutrition Handouts:
-
Visit Nutrition Education Materials | Alberta Health Services for provincial handouts to support patient education. Refer to section “Kidney Disease.”
-
A Renal Nutrition Guideline is also available to provide health professionals an overview of evidence-based nutrition recommendations for adults with chronic kidney disease. Nutrition & Disease Management | Alberta Health Services
Kidney Foundation of Canada: Kidney Foundation
BC Renal Agency: http://www.bcrenal.ca