The focus of this learning module for health educators is the management of diabetes in stages 4 and primarily stage 5 chronic kidney disease (CKD). Diabetes management in stages 1-3 CKD differs little from that of diabetes management without renal disease.
- Stage 1: GFR > 90
- Stage 2: GFR 60-89
- Stage 3: GFR 30-59
- Stage 4: GFR 15-29
- Stage 5: GFR < 15 or dialysis (End Stage Renal Disease – ESRD)
Diabetes is the most common cause of end-stage renal disease (ESRD) and dialysis, but individuals can have CKD and ESRD from other causes and have diabetes as an additional comorbidity.
What is Chronic Kidney Disease (CKD) and Diabetic Nephropathy?
- First of all, it may be helpful to review basic kidney function. Kidneys are vascular organs composed of about a million tiny units called nephrons. The nephron, magnified in picture below, is composed of a tiny filter called the glomerulus and an attached tubule. As blood flows through the glomerulus the first stage of filtration occurs where larger molecules such as proteins are prevented from passing into the filtrate. The filtrate then passes through the tubule where electrolytes, glucose, and amino acids may be reabsorbed into the blood. The tubule is where SGLT-2 receptors and to a much lesser extent SGLT-1 receptors function to drive glucose reabsorption. (Click here to learn more about SGLT inhibitor medications). Waste products such as urea, uric acid, creatinine and extra fluid remain in the filtrate that is sent to the collecting tubule as urine. Urine passes through the ureters to the bladder.
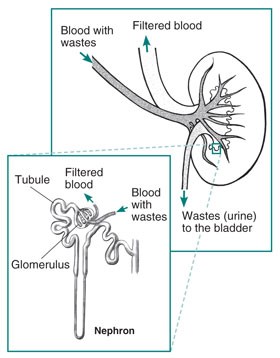
Photo courtesy of: https://www.niddk.nih.gov/health-information/kidney-disease/kidneys-how-they-work
- Along with removing waste and extra fluid from the blood, kidneys have an important role in keeping electrolytes such as sodium, potassium and phosphate levels stable. Additionally, the kidneys play a role in the generation of important hormones that help regulate blood pressure (through the enzyme renin), in making red blood cells (erythropoietin) and in maintain bone health (calcitriol/Vitamin D).
Chronic Kidney Disease (CKD)
- Chronic Kidney Disease (CKD) encompasses a variety of kidney diseases. People with diabetes can experience diabetic nephropathy, nephropathy secondary to vascular damage from hypertension, and other renal problems not related to diabetes such as polycystic kidney disease. Chronic Kidney Disease is defined as the following, according to http://www.ckdpathway.ca/aboutckd:
- eGFR < 60 mL/min/1.73m2 that is present for ≥ 3 months
- - or –
- Markers of kidney damage present for ≥ 3 months:
- Albuminuria ≥ 3 mg/mmol
- Urine sediment abnormalities
- Structural or pathological abnormalities
- Refer to table Diabetes Canada 2018 Guidelines for Stages of Chronic Kidney Disease:

Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes. 2018;42(Suppl 1):S1-S325. Accessed May 2018.
Diabetic Nephropathy
- Kidney damage caused by diabetes is a complex, multifaceted disease thought to be caused by both metabolic and genetic factors. Chronic hyperglycemia is linked to intraglomerular hypertension, glomerular damage and hypertrophy, altered permeability of glomerular membranes and atherosclerosis of glomerular vessels. We often simplify the explanation for patients by stating that high blood sugars can result in damage to the filters in our kidneys so that they can no longer filter our blood properly. This causes beneficial proteins to leak into the urine and waste products to build up in the blood. Some clients relate to the following visual analogy:
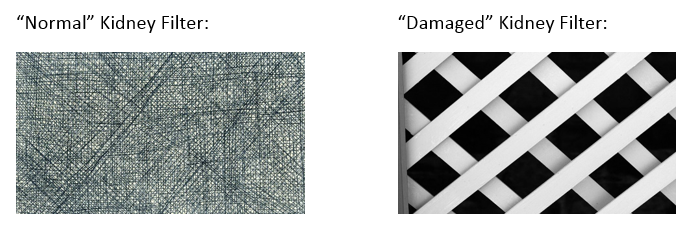
Pictures courtesy of Clip Art (have to reference?)
- Or, alternatively some refer to the analogy of a coffee filter when teaching the concept of nephropathy to clients. For example, high glucose levels and/or high blood pressure levels could act to damage the filters in the kidneys the same as putting a coffee filter under a tap running at full water pressure. The end result would be theoretical “holes” causing items to leak through when the filter is used. The item leaking through would be albumin. This would be one of the first indicators of damage to the kidneys.
2. How is Diabetic Nephropathy Prevented and Screened for?
Primary Prevention
- Glycemic Control: The Diabetes Complications and Control Trial (DCCT), landmark study in type 1 diabetes population, showed that intensive glycemic control (A1c less than 7.0%) reduced albuminuria by 34% in those with no complications at beginning of study. The United Kingdom Prospective Diabetes Study (UKPDS), landmark study in type 2 diabetes population, showed that intensive glycemic control (A1c less than 7.0%) also reduced albuminuria risk by 34%. The main message is to promote the best blood sugars possible soon after diagnosis.
- Blood Pressure Control: According to 2018 Diabetes Canada CPG’s, optimal blood pressure likely helps to prevent diabetic nephropathy. However, blood pressure control is especially important in the prevention of other kidney diseases people with diabetes may be at risk of, in particular, ischemic damage secondary to vascular disease and hypertension. Target blood pressure is less than 130/80 mm Hg.
- Blockade of the renin-angiotensin-aldosterone system (RAAS)- ACE (Angiotensin Converting Enzyme) inhibitor or ARB (angiotensin II receptor blocker) medications have been shown to prevent diabetic nephropathy in those with diabetes and hypertension.
Secondary Prevention
- Glycemic Control: The DCCT showed that intensive glycemic control lowered albuminuria by 43% and proteinuria by 56% in those with pre-existing kidney complications.
- Blood Pressure Control: The 2018 Diabetes Canada CPG’s state renal damage can be slowed by optimization of blood pressure. Target blood pressure is less than 130/80 mm Hg.
- Blockade of RAAS: ACE inhibitor and ARB medications can slow progression of diabetic nephropathy independent of blood pressure level. This means that even without diagnosis of hypertension, if albuminuria is present, blockade of RAAS has been shown to slow kidney damage.
- SGLT2 inhibitors: In patients who have CKD and proteinuria, SGLT2 inhibitors can slow progression of renal disease (strongest evidence is in those with eGFR 30-90 mL/min/1.73m2and heavy proteinuria with ACR > 33.9 mg/mmol).
Screening
Screening for CKD should begin upon diagnosis and then annually in type 2 diabetes. Screening can be delayed for 5 years after diagnosis in type 1 diabetes, and then annually thereafter.
- Random Urine ACR (Albumin to Creatinine Ratio): As explained by http://www.ckdpathway.ca/aboutckd, random urine ACR is preferred to detect proteinuria as it has greater sensitivity than protein:creatinine ratio (PCR) for low levels of proteinuria. Excess amounts of protein in urine are a marker of kidney damage. If the random ACR is abnormal (greater than 2.0 mg/mmol), 2 more repeat tests should be completed in next 3 months. If 2/3 ACR’S are greater than 2.0 mg/mmol, CKD is diagnosed. The exception to this, would be in the case of ‘overt nephropathy’ in that the random urine ACR is greater than 20 mg/mmol and CKD diagnosed immediately.As listed in the 2018 CPG’s, albuminuria can be temporarily increased in the following situations:
- Recent major exercise
- Urinary tract infection
- Febrile illness
- Decompensated heart failure
- Menstruation
- Acute severe elevation in blood glucose
- Acute severe elevation in blood pressure
- Creatinine for eGFR: As explained by http://www.ckdpathway.ca/aboutwhoandhowtotest, eGFR is the best measure of kidney function. eGFR <60mL/min/1.73m2 for ≥ 3 months is diagnostic for CKD.
3. CKD Stages 4 and 5 and Treatment Overview - Key Messages
The focus of this learning module for health educators is the management of diabetes in stages 4 and 5 chronic kidney disease (CKD). Diabetes management in stages 1-3 CKD differs little from that of diabetes management without renal disease.
- Stage 1: GFR > 90
- Stage 2: GFR 60-89
- Stage 3: GFR 30-59
- Stage 4: GFR 15-29
- Stage 5: GFR < 15 or dialysis (End Stage Renal Disease – ESRD)
Diabetes is the most common cause of end-stage renal disease (ESRD) and dialysis, but individuals can have CKD and ESRD from other causes and have diabetes as an additional comorbidity.
The key differences in the management of diabetes with stage 4 or 5 CKD, compared to management with stage 1-3 CKD, are outlined in this section. Please see section 4 below for a more thorough explanation of these differences.
Hypoglycemia
Individuals with advanced CKD on insulin or insulin secretagogues are at increased risk of hypoglycemia, particularly overnight and in the fasting state.
- Contributing causes include:
- Reduced clearance of medications/insulin
- Decline in renal mass and impaired renal gluconeogenesis
- Dietary factors: uremia increases anorexia/nausea/vomiting; suboptimal nutrition
- Other possible factors: gastroparesis, hypoglycemia unawareness secondary to autonomic neuropathy
- Notes:
- As patients on insulin progress to ESRD, their insulin requirements drop and some with Type 2 diabetes may no longer require insulin
- Some may experience significant drops in overnight blood glucose readings without evening insulin injections
- Different methods of dialysis will impact the risk of hypoglycemia
A1c
Home glucose monitoring is a more reliable marker of glycemia than A1c in late stages CKD.
- A1c can be overestimated with iron, folate or vitamin B12 deficiency.
- A1c can be underestimated with decreased RBC lifespan seen in ESRD (uremia), hemodialysis and with the replacement of erythropoietin (EPO) and iron.
- Glycated fructosamine and glycated albumin are not recommended in ESRD outside of a research setting.
Glycemic Targets
Glycemic targets should be individualized. This population of patients often have multiple comorbidities and should be considered vulnerable. The primary goal is to avoid hypoglycemia and significant hyperglycemia.
Targets for those on renal replacement therapy:
- CBG ac meals: <8.0 mmol/L
- CBG 2 h pc meals: < 12.0 mmol/L
- A1c: 7.1-8.5%
Non-insulin antihyperglycemic agents
- Medications should be prescribed and adjusted to suit individual patient glucose patterns, lifestyle and comorbidities.
- Some antihyperglycemic agents require dose reductions as the eGFR declines, and most are discontinued in stage 5 CKD.
- Sulfonylureas are usually discontinued given reduced clearance and risk of hypoglycemia.
- Select antihyperglycemic agents may be continued depending on the eGFR and other comorbidities; these include SGLT2 inhibitors, GLP-1 receptor agonists, DPP4 inhibitors, repaglinide and metformin, if deemed appropriate in individual patients.
Insulin
- Starting insulin doses are usually less in stage 4-5 CKD compared to stage 1-3. Please refer to the Insulin Formulas page or www.bbit.ca.
- As patients progress to ESRD, their insulin requirements drop significantly and some with Type 2 diabetes may no longer need insulin, while others may not need evening basal insulin.
- A basal bolus insulin therapy (BBIT) regimen with regular home glucose monitoring may be considered the safest regimen, with analogue insulin chosen over intermediate insulin (NPH) when there is recurrent hypoglycemia.
- For patients on kidney replacement therapy, insulin doses may vary depending on the day (hemodialysis vs. non-hemodialysis days) or on the concentration of glucose in the dialysate (especially with peritoneal dialysis).
- Meal bolus insulin timing should be matched to the patient’s rate of digestion. In the case of delayed gastric emptying due to autonomic neuropathy (gastroparesis), rapid insulin may be given after the meal or regular insulin may be given pre-meal, depending on the degree of delayed digestion.
Nutrition
- Referral to a Registered Dietitian for nutrition counselling to ensure nutritional adequacy of the diet is recommended for patients with declining kidney function (see Renal Nutrition Pathway DCC).
-
Please see the specific factors related to HD and PD (links following). Here are some additional nutritional considerations:
-
A bedtime snack is often recommended in stages 4-5 CKD for individuals on insulin or insulin secretagogues, even in the absence of injected evening basal insulin, to prevent hypoglycemia.
-
Glucose from dialysate can impact blood glucose levels and weight, as previously discussed. This is of greater concern in peritoneal dialysis.
-
Patients with gastroparesis should be encouraged to eat smaller, frequent, low-fat, and low-fibre meals.
-
The management of ESRD will require that some foods or supplements be limited in sodium, potassium, phosphorus, fluid, or protein content depending on individual patient laboratory results.
-